We are a multidisciplinary group interested in understanding how tumors arise and how they progress to a lethal metastatic disease. In particular, our research focuses on (1) understanding the molecular mechanisms underlying the progression of metastatic ovarian cancer, (2) identifying new therapeutic targets and (3) developing novel strategies to treat this deadly disease. To seek answers to these questions, we employ a broad range of state-of-the-art techniques and tools such as CRISPR-Cas9 gene editing, mass spectrometry, fluorescent imaging, “omic” analyses, flow cytometry, fluorescence activated cell sorting, high-throughput drug screening platforms, and in vivo tumor modeling in mice. There are several exciting research projects that are currently ongoing in our laboratory. Some of the key areas of focus are described in brief below.
I. FACTORS DRIVING OVARIAN CANCER PROGRESSION
A major focus in the Padmanabhan lab is to understand the molecular, genetic, and biochemical mechanisms underlying the initiation and metastatic progression of ovarian cancer, the most lethal gynecological malignancy in the developed world. Over 90% of high-grade serous ovarian cancers have mutation in the tumor suppressor gene p53. Majority of these mutations are gain-of-function mutations that converts the short-lived tumor suppressor p53 protein into a very stable oncoprotein that drives cancer progression and drug resistance. Current efforts in the laboratory focuses on understanding how specific p53 gain-of-function mutations impacts metastatic progression of ovarian cancer. We are also interested in elucidating factors that regulates the stability and levels of mutant gain-of-function p53 proteins in ovarian cancer cells.
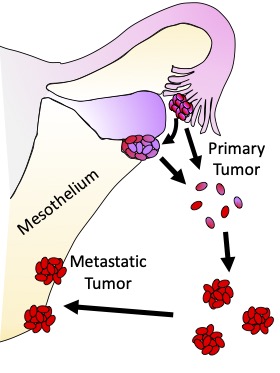
II. TUMOR MICROENVIRONMENT AND METASTASIS
The heterogeneity of cancer cells within a tumor and the dynamic nature of their interaction with each other, the stroma, and the immune cells present a daunting challenge to the development of effective therapies. We are interested in understanding the changes that happen within the cancer cells (cancer cell intrinsic) and in the tumor microenvironment (cancer cell extrinsic) during the various stages of tumor progression in response to different physiologic stimuli and stress. These studies utilize well-defined in vitro systems (engineered cell line models etc.), transgenic and xenograft in vivo mouse tumor models as well as tumor samples from human patients. Understanding how tumor cells remodel their microenvironment will help identify new factors that impact tumor progression.
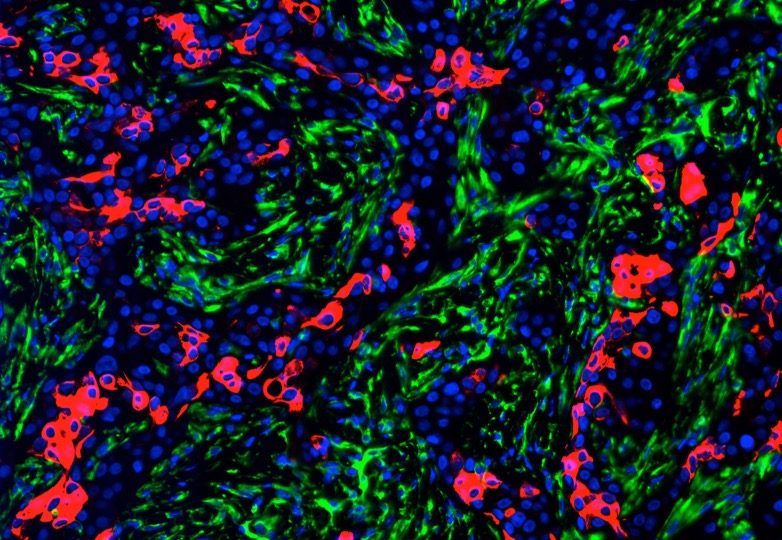
III. PROTEIN HOMEOSTASIS AND CANCER
The cellular protein quality control machinery is vital for maintaining protein homeostasis both under physiological conditions and during stress. Aberrations in protein quality control is implicated in a plethora of human diseases including cancer. Elevated proteasome activity is a hallmark of cancer cells and proteasome inhibitors are used as frontline therapeutic in several cancers. In our lab, we are interested understanding the link between protein homeostasis, proteotoxic stress, and cancer. Understanding how aberrations in protein quality control mechanisms impact cancer progression will identify new therapeutic targets and help develop more effective strategies to treat cancer.
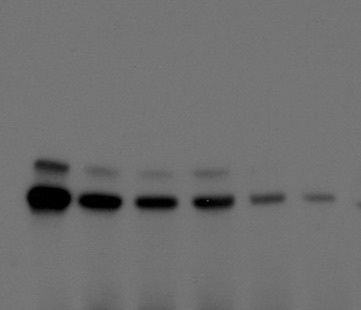
IV. THERAPEUTIC DEVELOPMENT
Another area of focus in our lab is to develop clinically translatable therapeutic strategies to treat metastatic tumors. Current studies focus on using high-throughput screens to identify novel small molecules to target key cancer signaling proteins and developing personalized approaches to treat metastatic ovarian cancer.